Aceclofenac EP Impurity E with high purity CAS 139272-67-6 CAS NO.139272-67-6
- FOB Price: USD: 50.00-50.00 /Gram Get Latest Price
- Min.Order: 10 Milligram
- Payment Terms: T/T
- Available Specifications:
purity 95%(0-1)Gram
- Product Details
Keywords
- Aceclofenac EP Impurity E
- Aceclofenac
- 139272-67-6
Quick Details
- ProName: Aceclofenac EP Impurity E with high pu...
- CasNo: 139272-67-6
- Molecular Formula: C18H17Cl2NO4
- Appearance: White to Light Yellow Solid
- Application: For lab research use only
- DeliveryTime: Package will dispatched in 7-15 days(A...
- PackAge: Moisture-proof packing
- Port: Shenzhen
- ProductionCapacity: 1 Gram/Week
- Purity: >95%
- Storage: Store at 2-8℃, dry, in dark
- Transportation: by air
- LimitNum: 10 Milligram
Superiority
Topbatt Chemical Co., Ltd., Established in 2019, located in Shenzhen, Guangdong Province, is a Manufacturer and Trading company which specialized in fine chemicals like Pharmaceutical Reference Standards and Stable Isotopes. Our Stable Isotopes product line including 2H Labelled APIs, Reagents and Intermediates, 13C Labelled Substance, 15N Labelled Substance and 18O Labelled Substance.
We own a number of Senior pharmaceutical synthetic engineer, analysis engineer and analytical testing instrument. It make us have more than 1700 varieties compouds with high purity in stock in a short time.
We can provide you not only stock items, but also Custom Synthesis Service.
All of our products provided with COA, HPLC/GC, HNMR, qNMR, MS Spectrum.
Contact us at 0086-18923794376 or email us at yehh@topbatt.net, 24/7 stand by for you.
After-sales issues will be settled in 24 hours.
Details
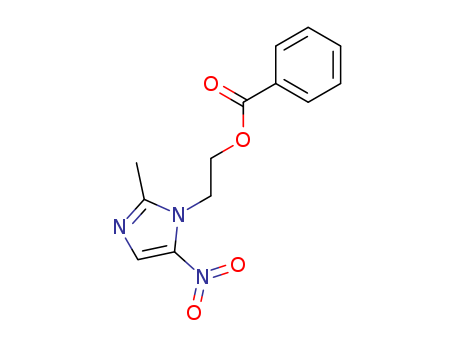
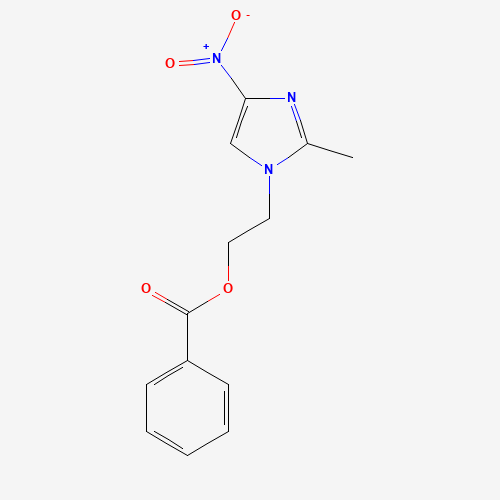
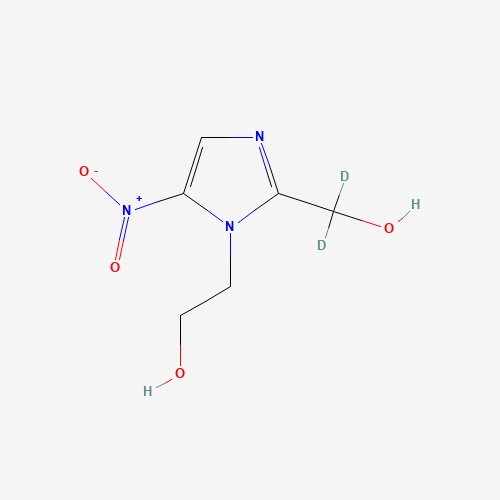
Product Name: Aceclofenac EP Impurity E
Chemical Name: 2-ethoxy-2-oxoethyl 2-(2-((2,6-dichlorophenyl)amino)phenyl)acetate
Molecular Formula: C18H17Cl2NO4
Molecular Weight: 382.24
CAS#: 139272-67-6
Purity: 95%
Price: Negotiable
Available Document: COA, HPLC/GC, HNMR, qNMR, MS Spectrum
Payment Term: Payment in advance by T/T.
Shipment: Package usally dispatched in 7-15 days(According to the date of payment) by air.
Trade Term: EXW, FOB, CFR, DDU, DDP, ect.
Aceclofenac EP Impurity E is an impurity of Aceclofenac. Aceclofenac is an oral non-steroidal anti-inflammatory drug (NSAID) with marked anti-inflammatory and analgesic properties used to treat osteoarthritis, rheumatoid arthritis and ankylosing spondylitis. It inhibits both isoforms of COX enzyme, a key enzyme involved in the inflammatory cascade. COX-1 enzyme is a constitutive enzyme involved in prostacyclin production and protective functions of gastric mucosa whereas COX-2 is an inducible enzyme involved in the production of inflammatory mediators in response to inflammatory stimuli. Aceclofenac displays more selectivity towards COX-2 (IC50 of 0.77uM) than COX-1 (IC50 of >100uM), which promotes its gastric tolerance compared to other NSAIDs. The primary metabolite, 4'-hydroxyaceclofenac, also minimally inhibits COX-2 with IC50 value of 36uM [2]. Although the mode of action of aceclofenac is thought to mainly arise from the inhibition of synthesis of prostaglandins (PGE2), aceclofenac also inhibits the production of inflammatory cytokines, interleukins (IL-1β, IL-6), and tumor necrosis factors (TNF)[1,2]. It is also reported that aceclofenac also affects the cell adhesion molecules from neutrophils [A19763]. Aceclofenac also targets the synthesis of glycosaminoglycan and mediates chrondroprotective effects [1].